SCS
-0.2000


New trials to evaluate IGC-AD1's potential impact on amyloid plaque, tau tangles, and cognitive decline in Alzheimer's disease
Expanded research positions IGC-AD1 as a potential treatment targeting the underlying disease pathology of Alzheimer's
New trials to evaluate IGC-AD1's potential impact on amyloid plaque, tau tangles, and cognitive decline in Alzheimer's disease
Expanded research positions IGC-AD1 as a potential treatment targeting the underlying disease pathology of Alzheimer's
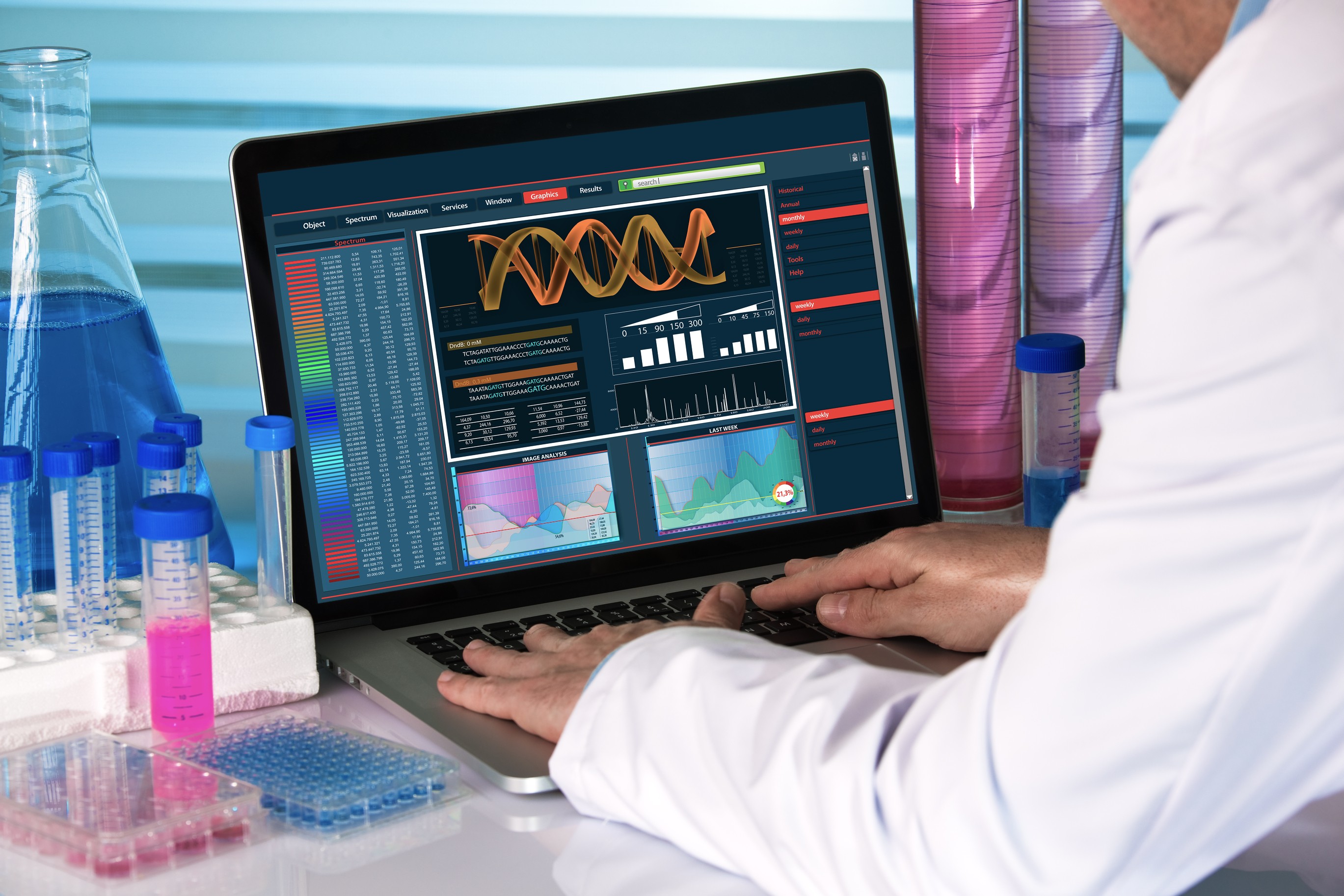
IGC Pharma, Inc. (NYSE American:IGC) ("IGC Pharma" or the "Company") announced today an expansion of its clinical research program for IGC-AD1, an investigational treatment for Alzheimer's disease. Building on Phase 2 interim results demonstrating reductions in agitation and cognitive improvement, the Company is initiating new trials to evaluate IGC-AD1's potential as a disease-modifying therapy.
The expanded research will explore how IGC-AD1's dual-action mechanism-combining anti-neuroinflammatory properties with amyloid- and tau-targeting effects-may slow the progression of Alzheimer's disease. These trials will evaluate critical outcomes, including cognitive function and biological markers associated with Alzheimer's, such as amyloid and tau levels, at multiple time points. Building on previously announced preclinical data showing IGC-AD1's impact on amyloid plaques and spatial memory, these investigations aim to explore its potential to influence key pathological features of Alzheimer's disease.
"We plan to initiate a new Phase 2 clinical trial for IGC-AD1 in 2025," said Ram Mukunda, CEO of IGC Pharma. "The trial underscores our commitment to advancing IGC-AD1 as a potential disease-modifying therapy for Alzheimer's. Cognitive decline is one of the most devastating aspects of Alzheimer's, severely impacting patients' memory, attention, and reasoning. By focusing on cognitive outcomes and underlying disease mechanisms such as amyloid plaques and tau tangles, we aim to address the critical unmet needs of patients and caregivers. This subsequent research phase is a significant step forward in delivering innovative treatments to those who need them most.
These new trials represent a pivotal step in advancing IGC-AD1 as a transformative Alzheimer's treatment. By exploring its potential as a disease-modifying therapy, we aim to create opportunities for strategic partnerships and licensing with major pharmaceutical companies. Our vision is to deliver innovative therapies that address the immense challenges faced by patients and caregivers while generating substantial value for our investors."
Building on Promising Preclinical Data
As previously reported, preclinical studies in Alzheimer's mouse models demonstrated an approximate 50% improvement in spatial memory, and cell line data showed a significant 20% reduction in amyloid aggregation following treatment. These preclinical findings, along with the interim Phase 2 data on cognition, provide a solid scientific basis for the expanded research program, which aims to validate IGC-AD1's ability to address key pathological features of Alzheimer's disease.
The ongoing 146-patient Phase 2 trial continues to enroll participants across the USA and Canada. With over 1,000 doses administered and no serious adverse events reported, the trial is on track to deliver comprehensive safety and efficacy data in 2025.
About IGC Pharma (dba IGC):
IGC Pharma is an AI-powered, clinical-stage biotechnology company focused on developing innovative treatments for Alzheimer's disease and transforming patient care with fast-acting, safe, and effective solutions. Our portfolio includes the TGR family, including TGR-63, which targets amyloid plaques, a hallmark of Alzheimer's. The IGC-C and IGC-M platforms are advancing in preclinical studies, focusing on metabolic disorders, tau proteins, early plaque formation, and multiple disease hallmarks. Our lead therapeutic candidate, IGC-AD1, is a cannabinoid-based treatment currently in a Phase 2 trial for agitation in dementia associated with Alzheimer's (clinicaltrials.gov, NCT05543681). Clinical data for IGC-AD1 demonstrated that it has the potential to transform patient care by offering faster-acting and more effective relief compared to traditional medications. Additionally, our AI models are designed to predict potential biomarkers for the early detection of Alzheimer's, optimize clinical trials, and predict receptor affinity, among others. With 28 patent filings and a commitment to innovation, IGC Pharma is dedicated to advancing pharmaceutical treatments and improving the lives of those affected by Alzheimer's and related conditions.
Forward-looking Statements:
This press release contains forward-looking statements. These forward-looking statements are based largely on IGC Pharma's expectations and are subject to several risks and uncertainties, certain of which are beyond IGC Pharma's control. Actual results could differ materially from these forward-looking statements as a result of, among other factors, the Company's failure or inability to commercialize one or more of the Company's products or technologies, including the products or formulations described in this release, or failure to obtain regulatory approval for the products or formulations, where required, or government regulations affecting AI or the AI algorithms not working as intended or producing accurate predictions; general economic conditions that are less favorable than expected; the FDA's general position regarding cannabis- and hemp-based products; and other factors, many of which are discussed in IGC Pharma's U.S. Securities and Exchange Commission ("SEC") filings. IGC incorporates by reference its Annual Report on Form 10-K filed with the SEC on June 24, 2024, and on Form 10-Q filed with the SEC on August 7, 2024, as if fully incorporated and restated herein. Considering these risks and uncertainties, there can be no assurance that the forward-looking information contained in this release will occur.
Contact Information
Rosalyn Christian / Walter Frank
IMS Investor Relations
[email protected]
(203) 972-9200
SOURCE: IGC Pharma, Inc.
P.McDonald--TFWP